REACTOR
FOR FORMING DIPHENYLAMINE
The reaction of aniline
forming diphenylamine and ammonia is a heterogen reaction where the reaction
occurs in the gas phase with the help of a solid metal oxide catalyst is mainly
alumina. Reaction mechanism is the reactants must be adsorbed on the catalyst
surface, after the reaction occurs at the surface of the catalyst, the desorption
substances from the surface of the catalyst and returned to the gas stream.
Reactions that occur
following equation as follows:
Reaction rate:
rA=
k.CA
rA = rate of
reaction , kmole/kg
cat.h
CA = concentration
of aniline , kmole/m3
k = overall
reaction rate constants , m3
/kg cat.h
We obtain the
necessary data contained in U.S. Pat. 3118944, after being processed then the value
will be the reaction rate constants as a function of temperature as follows::
T
= temperature, Kelvin
The reactor used is adiabatic Fixed bed Reactor that do not require cooling or heating means that the reaction was allowed to happen as it is. Although the exothermic reaction (heat out), but the heat that arises is not large enough to increase the reaction temperature remained still in the allowed temperature range.
The reactor used is adiabatic Fixed bed Reactor that do not require cooling or heating means that the reaction was allowed to happen as it is. Although the exothermic reaction (heat out), but the heat that arises is not large enough to increase the reaction temperature remained still in the allowed temperature range.
Data:
☺ the operating conditions
Temperature : 435-500°C
Pressure : ± 7.5 atm
Reactions heat : exothermic
Process conditions : adiabatic
Pressure : ± 7.5 atm
Reactions heat : exothermic
Process conditions : adiabatic
☺ Catalyst
Type : Alumina Al2O3
Shape : cylindrical
Size : 1/8 in x 1/8 in
Bulk
density, rB : 650 lb/ft3
Particle
density, rP : 1200
kg/m3
Arrangement of Differential Equations
If: x = C6H5NH2 conversion react
Arrangement of Differential Equations
If: x = C6H5NH2 conversion react
The composition of the mass in the reactor any
time:
Component
|
initial
|
Current
|
C6H5NH2
|
Fao
|
Fa = Fao - Fao.x
|
(C6H5) 2NH
|
Fbo
|
Fb =Fbo+½.Fao.x
|
NH3
|
Fco
|
Fc = Fco+½.Fao.x
|
C6H5NO2
|
Fdo
|
Fd = Fdo
|
Total
|
Fto
|
Ft = Fto
|
1. Mass balance of reactor
C6H5NH2 mass balance in the reactor in a volume elementRate of mass input – rate of mass output - rate of reaction = rate of accumulation
Mole C6H5NH2 any time :
Fa = Fao - Fao.x
2.
Heat balance of reactor
In a volume element:
Rate of heat
input – rate of heat output - rate of reaction heat = rate of accumulation
ΔHRT = heat of reaction as a function of temperature
Heat of reaction
Heat of formation of compounds at°C, ∆Hf
25 (Reid,
R.C, 1977)
Component
|
ΔHf (kcal/mole)
|
C6H5NH2
(C6H5) 2NH
NH3
|
20.76
31.07
-10.92
|
Gas heat
capacity, kcal/Kmole.K :
Data from (Reid, R.C., 1977) cp = a’ + b’.T + c’.T2
+ d’.T3 ; is converted into
cp = a + b.T
component
|
a
|
B
|
C6H5NH2
|
7.2898
|
0.065673
|
(C6H5) 2NH
|
5.7820
|
0.122340
|
NH3
|
6.2399
|
0.007591
|
C6H5NO2
|
5.0223
|
0.069627
|
Heat of reaction is calculated based on heat
of formation of products and reactants
Reaction
2 C6H5NH2
--------> (C6H5)2NH +
NH3
∆Hr25 =
∑∆Hf products - ∑∆Hf
reactants
∆Hr25 = ∆Hf (C6H5)2NH
+ ∆Hf
NH3 – ∆Hf
C6H5NH2
= 31.07 + (–10.92) -
2. (20.76)
= -21.37
kcal/2 mole
=
-21370 kcal/2 kmole C6H5NH2
=
-10685 kcal/ kmole C6H5NH2
Heat of reaction at temperature T:
(C6H5)2NH NH3
2 C6H5NH2 Δcp
Δa: 5.782 +
6.2399 - 2 (7.2898) = -2.5577
Δb: 0.12234 + 0.007591
- 2 (0.065673) = -1.4153.10-3
|
Δcp
= (-2.5577 - 1.4153.10-3 T) kcal/ kmole.K
= - 2.5577 (T-298) - 7.0765.10-4 (T2 -2982) kcal/2 kmole C6H5NH2
= - 2.5577 (T-298) - 7.0765.10-4 (T2 -2982) kcal/2 kmole C6H5NH2
= - 1.27885 (T-298) - 3.538.10-4
(T2 -2982) kcal/kmole C6H5NH2
Then:
ΔHrT = -10685 - 1.27885
(T-298) - 3.538.10-4 (T2 -2982) kcal/kmole
3.
Pressure
drop
Pressure drop is calculated using equation (5-196) Perry 1984:
With:
fm = friction factor, function of Reynolds number
n = exponential, function of Reynolds
number
Dp = equivalent diameter of catalyst, m
ε = voidage, empty volume fraction
φ = particle shape factor
G = mass velocity of fluid is based on
an empty tube (kg/h.m2)
ρ = fluid density, kg/m3
gc = 9.8 m/s2
Looking for a unit dP/dz be obtained:
The catalyst bed thickness calculation
to calculate the catalyst bed thickness in the reactor in order to obtain the desired conversion then use the following equations:
To obtain the desired conversion then 3 of the above differential equations solved simultaneously by using existing mathematical methods and computer programs are made.
Completion Ordinary Differential Equations (ODE) can be solved using the method:
1.Metode Euler (explicit), also called the method of integration of initial value
2.Metode Modified Euler (implicit) called the Predictor Corrector Method or Heun method
3.Metode Runge-Kutta of order of four
Step by step of calculation:
1. Determine the initial
condition into the reactor at z = 0 m:
x, T, P, Δz
2. Calculated heat
of reaction and reaction rate.
3. Calculated the value
of :
4. Calculation is
continued until the desired conversion.
The calculations above are done by trying variables that
can be varied as follow: Di, To and P
Another equation is needed physical properties as
function of temperature:
(Carl L. Yaws, 1999)
Component
|
BM
|
C6H5NH2
|
93.129
|
(C6H5) 2NH
|
169.227
|
NH3
|
17.031
|
C6H5NO2
|
123.113
|
Gas viscosity
Gas viscosity as
function of temperature in the table 21-1 and 21-2 Carl L. Yaws, 1999:
µ = A + B.T + C.T²
µ =
viscosity, µP (micro poise)
T =
Kelvin
1 µP = 0.00036
kg/h.m
A, B and
C constanta as follows :
component
|
A
|
B
|
C
|
C6H5NH2
|
-19.148
|
0.3067
|
-5.3256E05
|
(C6H5) 2NH
|
-21.162
|
0.2544
|
-4.5847E-05
|
NH3
|
-7.874
|
0.3670
|
-4.470E-06
|
C6H5NO2
|
-19.512
|
0.2958
|
-5.1687E-05
|
Viscosities of gas mixture
µm = ∑ µi,yi
Feed composition into the reactor by mass balance calculation is as follows:
µm = ∑ µi,yi
Feed composition into the reactor by mass balance calculation is as follows:
Component
|
Kg/h
|
Kmole/h
|
C6H5NH2
|
19654.32
|
211.0441
|
(C6H5) 2NH
|
63.13
|
0.4727
|
C6H5NO2
|
58.19
|
0.3731
|
Total
|
19775.65
|
211.8898
|
Quantity that are calculated
Quantity that
counted in the calculation of the reactor is:
1). Changed
in conversion
2). Changes
in reaction temperature
3). Changes
in pressure drop in the reactor
The program is run
using QuickBasic (QB) or Scilab.
The results run
the program will be obtained
relationship between the conversion to
catalyst bed thickness, reaction temperature to catalyst bed thickness and mass
composition as follows:
------------------------------------------------------------------------------------
z(m)
X FA FB FC T(°C) P(atm)
------------------------------------------------------------------------------------
0.00
0.0000 211.044 0.373 0.000
435.0 7.500
0.04
0.0020 210.612 0.589 0.216
435.4 7.498
0.08
0.0041 210.177 0.807 0.434
435.9 7.496
0.12
0.0062 209.738 1.026 0.653
436.3 7.494
0.16
0.0083 209.295 1.247 0.874
436.7 7.491
0.20
0.0104 208.849 1.471 1.098
437.2 7.489
0.24
0.0125 208.399 1.695 1.322
437.6 7.487
0.28
0.0147 207.946 1.922 1.549
438.1 7.485
0.32
0.0168 207.488 2.151 1.778
438.5 7.483
0.36
0.0190 207.027 2.382 2.009
439.0 7.481
0.40
0.0212 206.562 2.614 2.241
439.5 7.479
0.43
0.0235 206.093 2.849 2.476
439.9 7.476
0.47
0.0257 205.619 3.085 2.712
440.4 7.474
0.51
0.0280 205.142 3.324 2.951
440.9 7.472
0.55
0.0302 204.661 3.565 3.192
441.3 7.470
0.59
0.0325 204.175 3.807 3.434
441.8 7.468
0.63
0.0349 203.686 4.052 3.679
442.3 7.466
0.67
0.0372 203.192 4.299 3.926
442.8 7.463
0.71
0.0396 202.693 4.549 4.175
443.3 7.461
0.75
0.0420 202.190 4.800 4.427
443.8 7.459
0.79
0.0444 201.683 5.054 4.681
444.3 7.457
0.83
0.0468 201.171 5.310 4.937
444.8 7.455
0.87
0.0492 200.654 5.568 5.195
445.3 7.452
0.91
0.0517 200.133 5.829 5.456
445.8 7.450
0.95
0.0542 199.606 6.092 5.719
446.3 7.448
0.99
0.0567 199.075 6.357 5.984
446.9 7.446
1.03
0.0593 198.539 6.625 6.252
447.4 7.444
1.07
0.0618 197.998 6.896 6.523
447.9 7.441
1.11
0.0644 197.452 7.169 6.796
448.5 7.439
1.15
0.0670 196.901 7.445
7.072 449.0 7.437
1.19
0.0697 196.344 7.723 7.350
449.6 7.435
1.22
0.0723 195.783 8.004 7.631
450.1 7.432
1.26
0.0750 195.215 8.287 7.914
450.7 7.430
1.30
0.0777 194.642 8.574 8.201
451.2 7.428
1.34
0.0805 194.064 8.863 8.490
451.8 7.426
1.38
0.0832 193.480 9.155 8.782
452.4 7.424
1.42
0.0860 192.890 9.450 9.077
453.0 7.421
1.46
0.0888 192.294 9.748 9.375
453.5 7.419
1.50
0.0917 191.692 10.049 9.676
454.1 7.417
1.54
0.0946 191.084 10.353
9.980 454.7 7.415
1.58
0.0975 190.470 10.660 10.287
455.3 7.412
1.62
0.1004 189.850 10.970 10.597
455.9 7.410
1.66
0.1034 189.223 11.283 10.910
456.5 7.408
1.70
0.1064 188.590 11.600 11.227
457.2 7.406
1.74
0.1094 187.950 11.920 11.547
457.8 7.403
1.78
0.1125 187.304 12.243 11.870
458.4 7.401
1.82
0.1156 186.651 12.570 12.197
459.1 7.399
1.86
0.1187 185.990 12.900 12.527
459.7 7.396
1.90
0.1219 185.323 13.234 12.861
460.3 7.394
1.94
0.1251 184.648 13.571
13.198 461.0 7.392
1.98
0.1283 183.966 13.912 13.539
461.7 7.390
2.01
0.1316 183.277 14.256 13.883
462.3 7.387
2.05
0.1349 182.580 14.605 14.232
463.0 7.385
2.09
0.1382 181.876 14.957 14.584
463.7 7.383
2.13
0.1416 181.163 15.313 14.940
464.4 7.380
2.17
0.1450 180.443 15.674 15.301
465.1 7.378
2.21
0.1485 179.714 16.038 15.665
465.8 7.376
2.25
0.1519 178.978 16.406 16.033
466.5 7.373
2.29
0.1555 178.232 16.779 16.406
467.2 7.371
2.33
0.1590 177.479 17.156 16.783
468.0 7.369
2.37
0.1627 176.716 17.537 17.164
468.7 7.366
2.41
0.1663 175.945 17.923 17.550
469.5 7.364
2.45
0.1700 175.164 18.313 17.940
470.2 7.362
2.49
0.1738 174.375 18.708 18.335
471.0 7.359
2.53
0.1775 173.576 19.107 18.734
471.7 7.357
2.57
0.1814 172.767 19.511 19.138
472.5 7.355
2.61
0.1852 171.949 19.920 19.547
473.3 7.352
2.65
0.1892 171.121 20.335 19.962
474.1 7.350
2.69
0.1931 170.283 20.754 20.381
474.9 7.347
2.73
0.1972 169.434 21.178 20.805
475.7 7.345
2.77
0.2012 168.575 21.607 21.234
476.6 7.343
2.80
0.2054 167.706 22.042 21.669
477.4 7.340
2.84
0.2095 166.825 22.482 22.109
478.2 7.338
2.88
0.2137 165.934 22.928 22.555
479.1 7.335
2.92
0.2180 165.031 23.380 23.007
480.0 7.333
2.96
0.2224 164.117 23.837 23.464
480.8 7.331
3.00
0.2267 163.191 24.300 23.927
481.7 7.328
3.04
0.2312 162.253 24.769 24.396
482.6 7.326
3.08
0.2357 161.303 25.244 24.871
483.5 7.323
3.12
0.2403 160.340 25.725 25.352
484.4 7.321
3.16
0.2449 159.365 26.212 25.839
485.4 7.319
3.20
0.2496 158.377 26.706 26.333
486.3 7.316
3.24
0.2543 157.376
27.207 26.834 487.3
7.314
3.28
0.2591 156.362 27.714 27.341
488.2 7.311
3.32
0.2640 155.334 28.228 27.855
489.2 7.309
3.36
0.2689 154.293 28.749
28.376 490.2
7.306
3.40
0.2739 153.237 29.277 28.904
491.2 7.304
3.44
0.2790 152.167 29.812 29.439
492.2 7.301
3.48
0.2841 151.082 30.354 29.981
493.3 7.299
3.52
0.2893 149.983 30.904 30.531
494.3 7.296
3.56
0.2946 148.868 31.461 31.088
495.4 7.294
3.59
0.3000 147.738 32.026 31.653
496.4 7.291
3.63
0.3054 146.592 32.599 32.226
497.5 7.289
3.67
0.3109 145.430 33.180 32.807
498.6 7.286
3.71
0.3165 144.252 33.769 33.396
499.7 7.284
3.75
0.3221 143.057 34.367
33.993 500.8
7.281
3.79
0.3279 141.846 34.972 34.599
502.0 7.279
3.83
0.3337 140.617 35.587 35.214
503.1 7.276
3.87
0.3396 139.371 36.210 35.837
504.3 7.274
3.91
0.3456 138.107 36.841 36.468
505.5 7.271
3.95
0.3517 138.101 36.844 36.471
506.7 7.268
--------------------------------------------------------------------------------------
Graphic relationship shown by the figure below:
☺Thank you for
visiting my blog

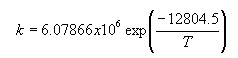














Tidak ada komentar:
Posting Komentar